Mini Bioreactor RTS-1C
Personal bioreactor provides Reverse-Spin® technology.
This provides a highly efficient mixture and oxygen supply for aerobic cultivation.
Combined with a near-infrared optical system it is possible to register cell growth kinetics non-invasively in real time.
Request a quoteMini-Bioreactor with Cooling
- The Reverse-Spin® mixing principle in 50 ml Falkon tubes enables high kLa values (h-1) of up to 450, which are essential for efficient aerobic cultivation.
- Individually controlled bioreactor accelerates optimization processes
- Possibility of cultivating microaerophilic and obligate anaerobic microorganisms (not strict anaerobic conditions).
- Reverse-Spin® mixing principle enables non-invasive biomass measurement in real time
- Near-infrared optical system makes it possible to register cell growth kinetics
- Free of charge software for storage, demonstration and analysis of data in real time
- Compact design with low profile and small footprint for personal application
- Temperature control for bioprocess applications
- Active cooling for rapid temperature control, e.g. for temperature fluctuation experiments
- Task profiling for process automatization
- Cloud data storage to remotely monitor the process of cultivation while at home or using
a mobile phone
Software Features
- Logging of cell growth in real time
- Graphical 3D representation of OD or growth rate over time and unit
- Pause option
- Save/Load option
- Report option: PDF and Excel
- Connect up to 10 units simulaneously to one PC
- Remote monitoring option (internet connection required)
- Cycling/Profiling options
- User manual calibration possibility for most cells
Typical Applications
- Fermentation real time growth kinetics
- Clone candidate screening
- Protein expression
- Temperature stress and fluctuation experiments
- Media screening and optimization
- Growth characterization
- Inhibition and toxicity tests
- Strain quality control
| Order No. RTS-1C | 103.4010 |
| Measurement range | 0–10 OD at 10–20ml volume (0–19 OD λ600 nm equivalent) 0–8 OD at 20–30ml volume (0–15.2 OD λ600 nm equivalent) |
| Measurement wavelength (λ) | 850 nm |
| Measurement precision | ±0.3 OD |
| Light source | NIR Light diode |
| Measurement periodicity per hour | 1 – 60 |
| Cultural media volume | 10 – 30 ml |
| Type of tube for aerobic cultivation | 50 ml tube with membrane filter (TubeSpin® Bioreactor 50, TPP®)* |
| Type of tube for anaerobic cultivation | 50 ml tube with membrane filter (TubeSpin® Bioreactor 50, TPP®)* *it is also possible to use other manufacturer tubes of the same type, e.g. Corning® 50ml Mini Bioreactor, but the device rotor must be modified. It is possible to request this modif.. |
| Temperature setting range | +4°C up to +70°C |
| Temperature control range | 15°C below ambient up to +70°C |
| Temperature stability | ±0.1°C |
| Speed control range | 50 – 2000 rpm |
| Max. number of units connected to the software | 10 |
| Display | LCD |
| Minimum PC requirements | Intel/AMD Processor, 1 GB RAM Windows Vista/7/8/8.1/10/11, USB 2.0 port |
| Optimal PC requirements | Intel/AMD Processor, 3 GB RAM Windows Vista/7/8/8.1/10/11, USB 2.0 port |
| Overall dimensions (W×D×H) | 130 × 212 × 200 mm |
| Weight | 2.2 kg |
| Input current/power consumption | 12 V DC, 3.3 A / 40 W |
| External power supply | Input AC 100-240 V 50/60 Hz, Output DC 12 V |
Why do we need new mixing principle in biotechnology - in fact there already are various shakers for flasks, disposable bags with wave mixing technology, falcon tubes with permeable gas membranes and bioreactors with various mixing principles?
You are correct, in reality, there are many types of reaction vessels and bioreactors that the market can offer for the cultivation of microorganisms, yet we think that RTS technology has its unique niche.
The most important issue when choosing a bioprocess technology is whether it is scalable. The impossibility of direct transfer of optimal conditions from thermoshakers to bioreactors became a common issue. Many engineers are in search for the middle ground, which is so that the cultivation in the bioreactor would be as simple and accessible as in shaker flasks and so that the process could be carried out in parallel from one hand, and on the other hand so that the bioprocess would be scalable. Lower aeration values using shaker flasks in comparison to bioreactors or, in other words, low oxygen mass transfer into the culture medium does not allow getting as high cell densities as quickly as possible like in bioreactors.
RTS-1 has a higher rate of oxygen transfer into the culture medium then shake flasks?
Yes, and from the point of view of its place in the market of disposable bioreactors this smart mini-bioreactor RTS-1 is unique. It occupies an intermediate link between shake flask reactors and typical bioreactors and laboratory bioreactors, while being similar to shake flasks because of the possibility of interdependent matrix cultivation, and high kLa values makes RTS-1 friendlier for further scalability.
The contradiction between the shaker and bioreactor way of realization of bioprocess implementation is not only in the impossibility of matching the data obtained but also that the principle of agitation (mixing to maximize the interaction of the reactants and microorganisms in the bioreactor) is quite different. Mass transfer, including oxygen saturation of the medium, the speed of incubation in a shaker incubator are incomparably lower than that in the bioreactor. Bioengineers of world's leading companies are in search of an intermediary, and there are hybrid solutions like installing bioreactor on a shaker or non-invasive monitoring of parameters of cultivation, but they are destined for failure because the most important parameter of bioprocess is mass transfer and shakers maximum kLa is 100 h-1 while average bioreactor kLa is 1000 h-1.RTS-1 oxygen mass transfer into the broth medium is up to 200 h-1
and RTS mixing technology can occupy an intermediate stage of macroevolution of bioprocess and can be between shaker-incubators and bioreactors because of high kLa values and scalability. Moreover, when optimization bioprocess is required by matrix type cultivation where study of several interdependent physicochemical factors is required in configurations like, 2x2 = 4 RTS, 3x3 = 9 RTS, 4x4 = 16RTS, 5x5 = 25RTS, and even 10x10 = 100 concurrent bioreactors, the RTS can be perfectly applied. This kind of RTS technology bioreactor amount is acceptably imaginable on a laboratory bench or in case of the need of higher biological safety in Laminar Cabinets class II and III and this kind of solution can be cost efficient and consequently, promising.
What other noninvasive mixing principles that are available on the market today?
Biosan produces most of devices for noninvasive mixing which in turn can be used for bioprocesses. Moreover, we would like to introduce a new mixing principle into this table called Reverse Spinning. In the future, we will develop multi-channel RTS devices with higher and smaller volumes. In our estimates, the mixing technology can be scaled up to 20 litres and scaled down to 5ml. With a possibility to add vital bioprocess parameters noninvasive measurement such as pH and pO2.
What is the mixing principle of RTS technology devices?
Initiation of the Vortex Type Mixing (VTM) and depth of the Vortex cave depend on 1) angular speed of RS–Reactor 2) time from initiating rotation of RS–Reactor 3) growth media viscosity 4) temperature. These parameters, also, determine the angular speed of rotating Vortex Layer (VL) and transition state from the Irrotational Vortex (IRV), when angular speed of the VL is proportional to the radius, to the Rotational Vortex, when the angular speed of the VL is the same and VL looks like a monolithic Vortex cavity, as shown below. Common rules regulating VTM processes may be stated as follows: the more time has passed since Vortex formation, the more obvious is a transition from IRV to the RV. The concept of the Reverse–Spin (RS) mixing is based on these assumptions.

What is the measurement principle of RTS technology devices? What is the measurable range of biomass in OD600nm units?
The essential elements of RTS-1 measurement system consist, as shown below, of a light source, a detector (photodiode), a falcon tube compartment in a rotating state. The falcon tube accelerates to 2000 min-1 creating a monolayer of cell suspension shortening the optical path of the sample. By shortening the optical path, it is possible to measure biomass in the range of 0-19 OD600nm at 10-20ml and 0-15.2 OD600nm at 10-30ml.

What effect does the intensity of mixing have on mass transfer coefficient (kLa)?
O2 kLa into the medium is extremely important indicator to assess the possible RTS-1 prospects to obtain high output growth rates of biomass.
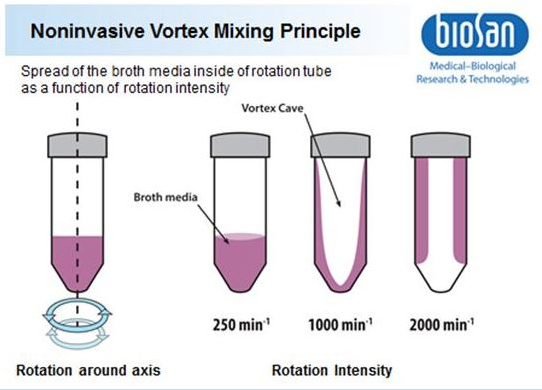
Stirring of the liquid medium is happening due to Vortex Type Mixing (VTM) and Reverse-Spin (RS). Due to multiple increase of the contact surface liquid and gaseous medium during the rotation and the emergence of waves (see fig. 1) at the time of movement direction change, the mixing efficiency is substantially increased both in liquid and liquid-gas phase. As a result, the saturation of the liquid phase by gas and its further dissolution in it occurs with greater efficiency than in most standard mixing devices.
As shown on Fig. 2, spread of broth media inside of the tube depends on rotation intensity, which in turn affects the efficiency of VTM and rate of O2 supply.

Why do you introduce a new standard of turbidity measurement in the near-infrared (850nm) spectra, and how to convert these results to more usual 600nm wavelength values?
Since we are dealing with a non-invasive method of measuring light scattering in cell suspension of growing cells, then the standardization of the vessel in which the fermentation takes place is very difficult. Instead of using a spectrophotometric cuvette and a spectrophotometer, we offer to use a standard falcon tube that is popular in growing microorganisms in the early stages of scale-up processes – a disposable falcon tube with respiratory membrane, which is slowly becoming a gold standard. Yet, falcon tubes are not 100% transparent and the degree of its turbidity depends on the temperature and the wavelength at which you are making the measurement. With this in mind, we have transitioned to NIR spectra wavelength to avoid the optical characteristics of the plastic material that is affected by temperature.
What is the conversion rate coefficient from 850nm to 600nm of factory calibration? How is it calculated?
Factory calibration of the instrument is designed for specific microorganism size of 0.4-0.8 x 1-3 μm and a cell volume of approximately 0.6-0.7 μm3. In case of exceeding the allowable size, the measurement system using factory calibration will not work correctly.
Optical density OD (λ = 850 nm) to OD (λ = 600 nm) conversion rate coefficient is equal to 1.9 (cells taken for measurement from stationary phase using a spectrophotometer and 1mm optical path cuvette).
Example of calculation: to convert 3.5 OD (λ = 850 nm) to OD (λ = 600 nm), multiply the result by 1.9, resulting in 6.65 OD (λ = 600 nm).
The microorganism that is used for factory calibration is E.coli BL21. The cells are taken from shake flask night culture at stationary phase of growth.
What can influence factory’s calibration measurement accuracy?
During the growth transition of Escherichia coli culture from the exponential growth to the stationary phase, a number of morphological and physiological changes take place, including cell volume decrease and cell shape change. Therefore, if cells taken for referent measurement using spectrophotometer at different stages from stationary phase then the correctness of measurement will be worse than specified.
How does the rotation intensity influence the temperature accuracy of the sample?
Firstly, Rotation intensity influences air transfer introduced to the tube from the outside environment. Secondly, the ambient temperature influences the temperature set in the bioreactor, and therefore can cool or heat the sample. Thirdly, contact between liquid and falcon tube compartment is less at higher rotation intensities. See image below, for breakdown of temperature differences (Δ t°C) between tube sample and set thermoblock temperature, depending on aerobe and microaerophilic rotation intensities at ambient temperature 23 °C ± 2 °C and 45% ± 10% RH.
The temperature profiling range of RTS-1C is quite high and because of dew point temperatures, moisture can appear on the optical axis of the measurement system and can influence the measurement results?
OD measurement accuracy is affected by moisture that can appear on the outside wall of the tube because of dew point temperature. Relative humidity and temperature affect the dew point, therefore the temperature of the tube must be higher than the dew point temperature for the measurement system to work correctly.
How to find optimal temperature profiling range to avoid dew point temperature?
Finding dew point temperature for the user.
Find the corresponding curve on image that matches the humidity of the room in which the device is located.
The horizontal axis indicates the temperature of the room in which device is located.
Using that information, make a projection on the vertical axis. The found point on the graph is the temperature of the dew point.

How to avoid dew point temperature during temperature profiling?
If the dew point temperature reached, moisture will disrupt correct measurement of the system. To avoid dew point temperature, decrease the temperature difference between the ambient temperature and bioreactor and sample temperature. By placing the bioreactor to an environmental chamber, the temperature difference can be significantly lower.
Example of calculation of environmental chamber temperature selection: If the range of temperature profiling is from +10°C to +40°C, calculate the environmental chamber temperature: (40-10) / 2 = 15°C.
Is it possible to place the device in a cold room or environmental chamber?
What is the cold room influence on temperature accuracy of the thermoblock?
It is possible to place the device in a cold room or environmental chamber, but the temperature of thermoblock and temperature of the sample will not be accurate at specific temperature ranges. Temperature measurements of the sample and corrections at maximum and minimum temperature ranges or 4°C-10°C and 60°C-70°C must be performed by the user. If any additional questions appear, please contact Biosan RTS support team directly for assistance.
How does the temperature influence the plastic material optical characteristics?
When temperature of the plastic material is changing, i.e. during temperature cycling from +37°C to +10°C and back every hour, the plastic material of the tube changes optical characteristics in a range of ±0.1 OD. This influence can be observed on all the ranges of temperature profiling that are exceeding 15°C or more.
How to create user calibration?
Get cell suspension samples in falcon tubes with typical optical densities of your experiments. If the maximal OD of your experiment (stationary phase) is 5 OD600nm then the recommended samples are 0 (ddH2O water or broth media) 1, 2, 3, 4, 5, 6 OD600nm. Volume accuracy of the samples must be ±0.05.
Measure OD at desired wavelength of each cell suspension using a spectrophotometer with proper prior dilutions. The proportionality between OD and cell density exists only for OD ≤ 0.4 (approximately), we recommend diluting samples to the range of 0.1-0.2 OD.
Multiply the dilution factor values to get the OD of the samples.
Proceed to the software calibration module described in the software manual.




